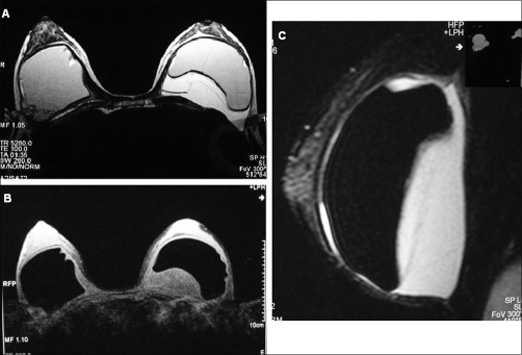
Prognosis if Treated Promptly
The standard approach to treatment of breast implant–associated anaplastic large-cell lymphoma (BIA-ALCL) is complete oncologic resection, as per the 2019 National Comprehensive Cancer Network (NCCN) consensus guidelines.
Results from a prospective study of 26 consecutive patients with confirmed BIA-ALCL between 2013 and 2018 show that en bloc surgical resection and explantation was curative in 100% of patients.
«When treated appropriately and in a timely fashion, BIA-ALCL has an excellent prognosis,» write Sarah E. Tevis, MD, assistant professor of surgery at the University of Colorado School of Medicine in Denver, and colleagues.
«Incomplete resections, partial capsulectomies, and positive margins are all associated with high rates of disease recurrence and, in rare cases, accelerated progression of disease,» they warn in a report published online February 27 in Aesthetic Surgery Journal.
The 2019 NCCN guidelines are based on long-term study findings confirming that surgical management provides superior event-free progression and overall survival compared to any other treatment modality, the authors point out.
However, they emphasize, en bloc resection «is indicated only for an established diagnosis of BIA-ALCL.»
A total capsulectomy, explantation, and removal of any associated masses or suspicious lymph nodes is not recommended «for merely suspicious or prophylactic surgeries,» the guidelines authors note.
Problem Only With Textured Implants
Tevis told Medscape Medical News that to date, BIA-ALCL has been confirmed only in women with textured implants. She pointed out that an initial report from the American Society of Plastic Surgeons (ASPS) patient registry supports this.
All 89 documented cases of BIA-ALCL submitted to the Patient Registry and Outcomes for Breast Implants and Anaplastic Large Cell Lymphoma Etiology and Epidemiology (PROFILE) registry between 2012–2018 were in patients implanted with textured devices.
A total of 96% of patients had local symptoms — most commonly periprosthetic fluid collection following implantation — and 9% had concurrent systemic symptoms. There were three deaths.
«We recommend that discussion about the risk of BIA-ALCL be included in the consent process for the placement of implants,» Tevis said. This includes «making patients aware of the disease, the presenting symptoms, and encouraging patients to seek medical attention if they develop symptoms,» she added.
As previously reported by Medscape Medical News, BIA-ALCL most commonly presents as a late onset lump or seroma in the capsule surrounding a textured breast implant filled with saline or silicone. Symptom onset occurs an average of 8 to 10 years after breast reconstruction following mastectomy or cosmetic breast enhancement.
Recently, a single case of fatal implant-associated ALCL was reported in a woman who underwent gluteal enhancement with textured implants.
Worldwide, the number of BIA-ALCL cases has been growing steadily. In the United States, the Food and Drug Administration (FDA) has received 457 unique medical device reports (MDRs) that met the pathologic criteria for BIA-ALCL between 2010 and September 2018. These included nine deaths.
Although 24 of these MDRs involved smooth implants, this information was «unverified,» says Mark W. Clemens, MD, associate professor of plastic surgery at the University of Texas MD Anderson Cancer Center in Houston. Clemens was also a co-author of the 2019 NCCN guidelines.
«The FDA confirmed that BIA-ALCL is predominantly associated with textured surface implants,» Clemens writes in a report on the ASPS website. «To date, no purely smooth-implant case of BIA-ALCL has ever been reported in any series, registry, or case report with a detailed history.»
Of the estimated 550,000 breast implants placed each year in the United States, about 70,000 are textured, a 12.7% market share that has remained stable for the past six years, according to Clemens. The ASPS estimates that the current lifetime risk of BIA-ALCL in US women is about 1 in 30,000.
Fatal Cases of BIA-ALCL
The ASPS is now aware of 688 unique cases of BIA-ALCL worldwide, says Clemens. These include 17 deaths in more than 30 countries.
Fourteen of these deaths resulted from respiratory failure following direct extension of BIA-ALCL into the chest wall, Clemens points out.
In most of these cases, diagnosis was delayed an average of 1 to 2 years from the onset of symptoms. None of the patients received complete surgical excision or targeted therapy.
«A multidisciplinary team approach is essential for the management of this uncommon malignancy,» write Clemens and co-authors of the 2019 NCCN guidelines.
«BIA-ALCL is generally an indolent and localized disease with excellent prognosis when patients receive surgical excision,» they write. «It remains unclear whether timely diagnosis can mitigate invasive disease or whether biologic variability of the tumor exists that affects prognosis.»
Routine sentinel node biopsy is unnecessary, and full axillary lymph node dissection is only indicated when multiple node involvement can be confirmed, the guideline authors say. However, they add, a «lymphoma oncology consultation and disease staging by imaging must be performed prior to surgery.»
Although research into the pathogenesis of BIA-ALCL is ongoing, results from a small study of 13 patients indicate genetic susceptibility factors may put some women at higher risk.
The study, led by Tevis, was published online on February 4 in the Aesthetic Surgery Journal.
In the study, patients who underwent testing for BIA-ALCL and human leukocyte antigen (HLA) polymorphisms were identified from 2017 to 2018.
Tevis and colleagues observed that the A26 allele occurred significantly more frequently in the general population compared with BIA-ALCL patients (0.2992 vs 0.07692, P < .001).
«This is early work in a small population of patients, but hopefully adds to our knowledge of what places patients at risk for developing the disease,» Tevis told Medscape Medical News.
«Other potential etiologies include mutations in the JAK-STAT pathway and chronic inflammation secondary to reaction to implant particulate, formation of biofilm, or autoimmune causes,» she said.
Although the risk of BIA-ALCL remains small, «we’re seeing more and more of it,» Tevis noted in a statement.
«Our hope is that by raising awareness of common presenting symptoms, proper treatment strategies and by continuing to build our understanding of the inner workings of BIA-ALCL, we can successfully treat the women who need treatment and, eventually, identify who is at highest risk for developing the disease,» she said.
Clinicians should report all confirmed cases of BIA-ALCL to the FDA’s Manufacturer and User Facility Device Experience (MAUDE) database, to the PROFILE registry, and to the implant manufacturer.
The FDA is holding a meeting about breast implants on March 25-26, 2019, and BIA-ALCL is one of the topics marked for discussion. Others include informed consent discussion between clinicians and patients that are going to have a breast implant.
Tevis disclosed no relevant financial relationships. Clemens reported that he is co-editor of the breast surgery section for the Aesthetic Surgery Journal and co-author of the 2019 NCCN guidelines for ALCL.
Asthet Surg J. Published online on February 27, 2019. Full text
Asthet Surg J. Published online on February 4, 2019. Full text
For more Medscape Oncology news, join us on Facebook and Twitter.