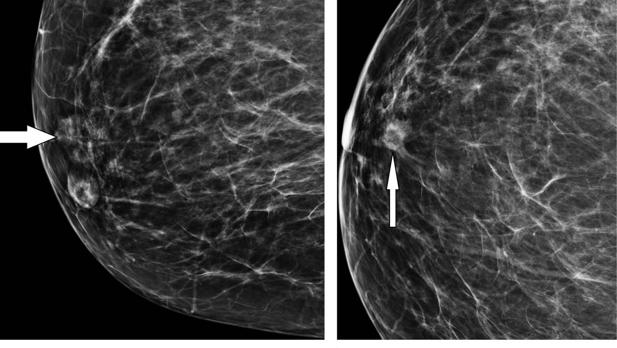
The United States Food and Drug Administration (FDA) has announced that it will allow continued sales of textured breast implants, which regulators in other nations have banned or restricted because of concerns about a rare cancer, breast implant- associated anaplastic large cell lymphoma (BIA-ALCL).
“At this time, the FDA does not believe that, on the basis of all available data and information, the device meets the banning standard set forth in the Federal Food, Drug and Cosmetic Act,” said Amy Abernethy, MD, PhD, FDA’s principal deputy commissioner, and Jeff Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health.
Some countries have taken stronger steps. Allergan said in December 2018 that it was suspending sales of textured breast implants in European markets, a day after French regulators announced that they would recall the product. As of March 2019, the company’s BioCell textured implants had been recalled in all 33 European countries, as well as Israel and Brazil, according to the FDA’s briefing for its March advisory committee on breast implants. In early April 2019, Health Canada was considering a ban on the products.
The FDA said that it will take steps to improve information about the risks associated with breast implants, which it lists as BIA-ALCL, the greater risk of BIA-ALCL with textured implants, and the risk of developing systemic symptoms.
The agency first warned about BIA-ALCL in 2011.
Concern over the issue has intensified in recent months, with the FDA noting in February 2019 that more cases had been reported, and in March 2019 the agency issued an update and held a 2-day meeting about the issue.
At that meeting, some researchers proposed that bacteria and biofilm on breast implants may cause BIA-ALCL, perhaps in relation to a sustained T-cell response.
In the latest statement, issued May 3, Abernethy and Shuren noted differences in the kinds of implants used in the US and international markets. Textured implants can account for as much as 80% of market share abroad, including products not used in the United States. In the United States, less than 10% of breast implants sold last year were textured implants.
“The type of macro-textured implants targeted by some of our international counterparts represents less than 5% of breast implants sold here,” Abernethy and Shuren said.
The FDA said it is considering methods for making physicians and consumers more aware of the risks of breast implants, including considering a boxed warning and a patient decision checklist. The agency also is looking at ways to address the complaints of women who have attributed their breast implants to chronic fatigue, cognitive issues, and joint and muscle pain.
“While the FDA doesn’t have definitive evidence demonstrating breast implants cause these symptoms, the current evidence supports that some women experience systemic symptoms that may resolve when their breast implants are removed, referred to by some patients and healthcare professionals as breast implant illness,” Abernethy and Shuren said.
Fuente: Medscape